The FDA is adding its most serious warning label to certain sleep aids after reports of fatalities and other dangerous side effects.
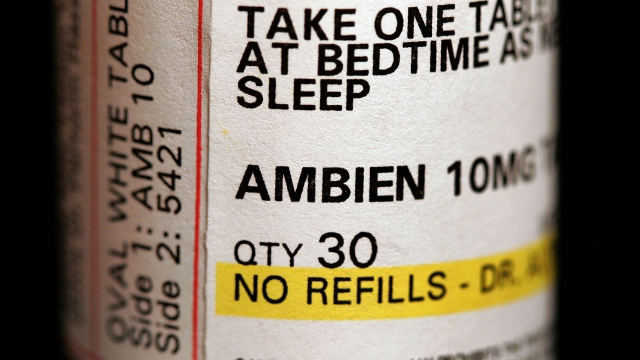
The agency announced Tuesday it'll require new "black box" warnings for the class of insomnia drugs that includes brand names Ambien, Lunesta and Sonata.
This is after a safety review confirmed reports of rare but serious injuries and deaths linked to complex sleep behaviors — like sleepwalking and sleep driving — after taking the medicines.
According to the FDA, 20 deaths and 46 non-fatal injuries were linked to the drugs in this latest review.
In 2007, the agency required a different type of warning label on certain sleeping pills, but the black box warning is its most prominent. It means clinical data has shown that reactions to the drug could result in injury or death.
Health regulators are also advising doctors not to prescribe the drugs to people who've experienced those types of side effects in the past.
In a CDC survey, around 4% of American adults said they had used prescription sleeping pills within the past month.
Additional reporting from Newsy affiliate CNN.